Technology / Business Introduction Let us introduce our technical capabilities.
We aim to solve human issues related to the environment and food problems and to realize a sustainable society by utilizing an original technology.
Electride catalyst
Prof. Hosono at Tokyo Institute of Technology realized the synthesis of electride (an electronized material), which electrons behave as negative ions for the first time in the world by using a cheap cement material (12CaO · 7Al2O3) (2003 Science magazine published : below "C12A7 Electride").
In addition, the Hosono Group applied the compound as a catalyst for synthesizing ammonia, thinking that the bond of nitrogen molecules could be easily cut off by utilizing the electron-donating property of C12A7 Electride, and it was found to be better than conventional catalysts. The results show that the activation energy is half the conventional way and the TOF*1 is one digit higher. (It was published in Nature Chemistry in 2012.*2)
As a result, it is currently attracting attention as a new catalyst that can synthesize ammonia at a lower temperature and lower pressure than the iron-based catalyst of the Haber-Bosch process. We aim to bring about ammonia supply chain innovation by developing a process that uses this electride catalyst and adapting it to the world.
*1 Turnover Frequency: Maximum number of molecules that can be converted into products per unit time at one catalyst site
*2 “Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store.” Nature Chemistry. https://www.nature.com/articles/nchem.1476
Advantages of Introducing Our Technology
Our company established an ammonia synthesis catalyst technology developed by the Hosono Group of Tokyo Institute of Technology and an on-site production technology that breaks the common sense of monopolar concentration and mass production of ammonia by designing a plant that matches the catalyst. It reduces transportation and storage costs and contributes to environmental load control and cost reduction.
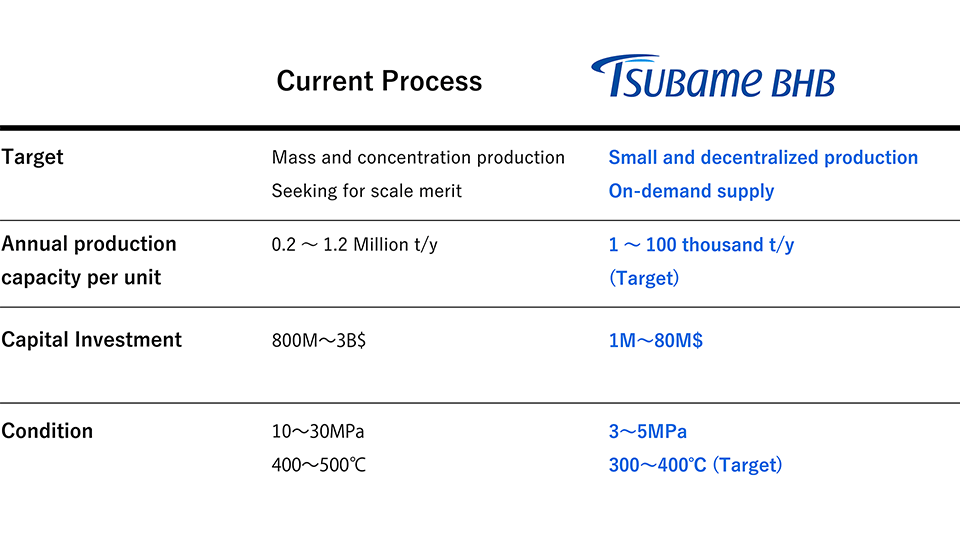
Comparison between existing and Tsubame BHB technology
Small Scale Onsite Ammonia Production Module using Tsubame BHB’s electride catalyst
Tsubame BHB Small Scale Onsite Ammonia System Pilot Plant
Green Ammonia and Fertilizer Project Brochure
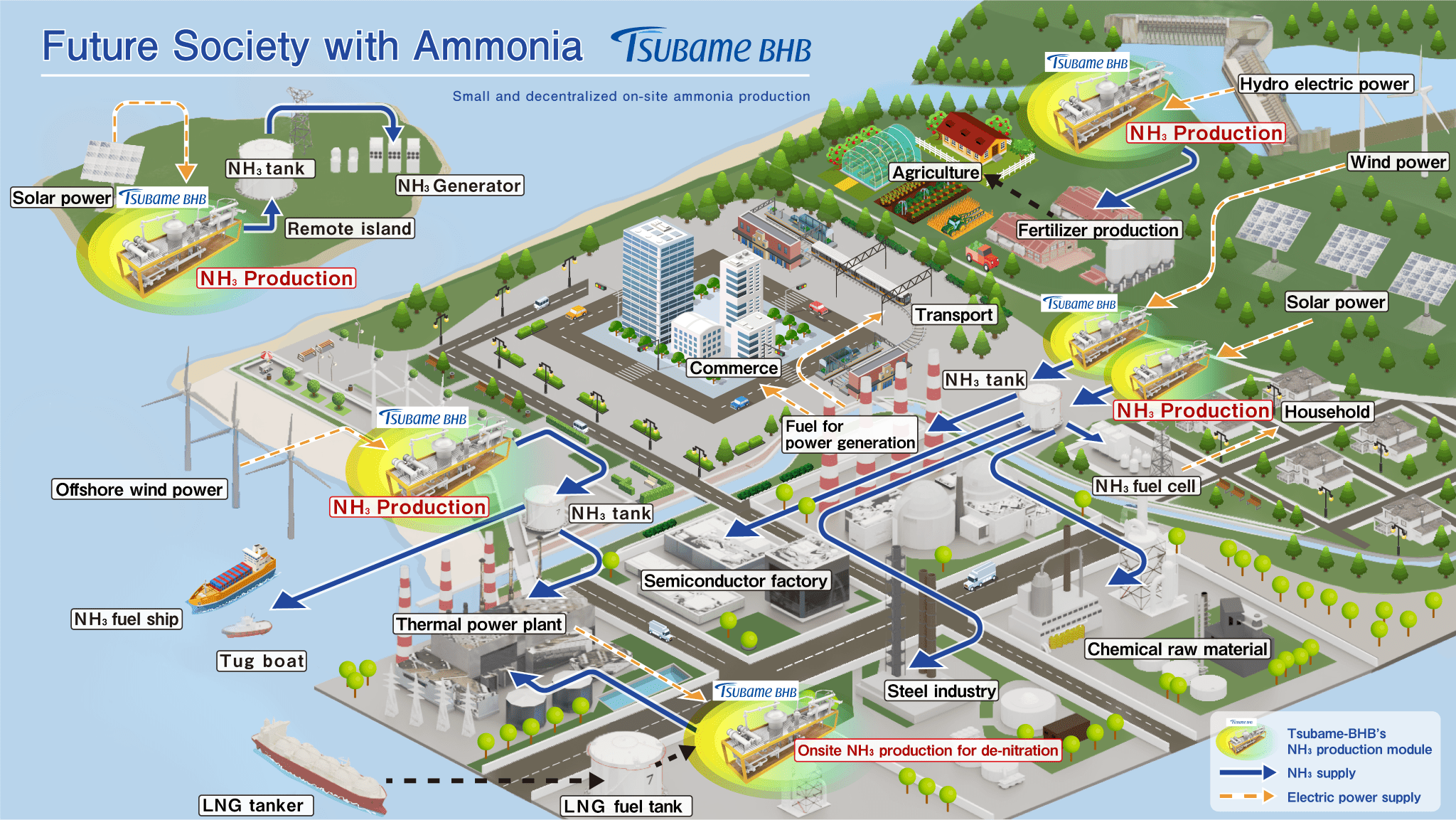
Future Society with Ammonia designed by Tsubame BHB
Examples of Utilizing Technology
Agricultural fertilizer application
More than 80% of ammonia is used as fertilizer. Many countries do not produce ammonia, and 120 countries and regions did not produce ammonia in 2015. Therefore, these countries are unable to install large and expensive current ammonia plants and are purchasing expensive ammonia and nitrogen-based fertilizers from abroad. We believe that the introduction of this technology will also contribute to those countries that need the nitrogenous fertilizers to produce crops efficiently.
Fermentation raw material application
Ammonia is used for amino acid production by fermentation. The amino group contains a nitrogen atom and is introduced in a form that supplements the lack of nitrogen during fermentation production. Unlike chemical synthesis methods, fermented amino acid factories are often located inland where sugar cane, cassava, etc., as the raw materials, are cultivated, so the ammonia produced in the coastal area is transported to their factory and used. Therefore, amino acids producers from such fermentations buy ammonia at a high price. By using this technology, only the ammonia required for the factory is produced on-site, leading to cost reduction.
Green ammonia applications (hydrogen carrier, etc.)
The recent rapid cost reduction of renewable energy is a great opportunity, and the utilization of ammonia as an energy and hydrogen carrier is drawing attention. There is much energy that cannot be fully utilized due to the lack of power lines, and we can propose a local production model for local consumption, which produces ammonia from water and air on the spot and uses it for various purposes such as chemicals and fertilizers. Our catalyst, which can be used at low pressure, is suitable for ammonia synthesis that uses electrolytic hydrogen generated at normal pressure. One of the merits is that it can be installed on the site near solar power generators or wind power generators, where small-sized plants are limited.
In addition, since the current ammonia synthesis generates a large amount of carbon dioxide during the production from hydrogen raw materials, synthesizing ammonia with green ammonia has the effect of suppressing carbon dioxide emissions.
Denitration applications for thermal power plants and refuse incinerators
At thermal power plants and refuse incinerators, ammonia is used to decompose NOxgenerated during incineration. If there is no ammonia plant near a thermal power plant or a refuse incinerator and it is transported from a distance, customers will be purchasing ammonia at a very high cost. By using our technology, it is possible to produce ammonia according to the amount used in the plant on the customer's premises and contribute to cost reduction. In the case of a thermal power plant, LNG, coal, or electrolytic hydrogen produced from surplus electricity can be used as a hydrogen source.
High purity ammonia for semiconductors
Ammonia is used in the production of semiconductor nitride films, etc. Although the amount of ammonia used for this semiconductor is not large, very high purity is required and it is purchased at a price of 20 to 50 times the market price. If our technology is used, there is a possibility that high-purity ammonia can be produced in a semiconductor factory, which will lead to a large cost reduction.
Relationship between Tokyo Institute of Technology and Ammonia Synthesis
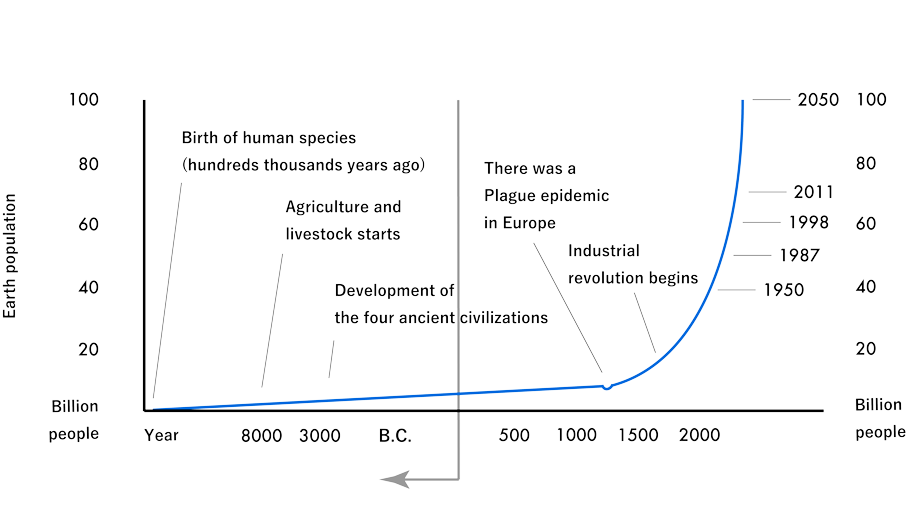
The world population is over 7 billion. Of these, "About 1 billion people are fed by Tokyo Institute of Technology." It is likely to be scolded as a grand delusion, but it is not a baseless story.
By the 1950s, it was thought that the planet could support at most 3 billion people, but in the 1950s it reached 2.5 billion. (ref. the figure below) Without the efforts of the people related to the Tokyo Institute of Technology, the population of the earth would have been less than half of what it is now, and the fight for food would have been extremely fierce. Food is at the top of what is essential to our survival. The nitrogen fertilizer essential for its food production relied on Chile's niter.
When it began to be depleted, the famous Haber Group succeeded in industrially producing ammonia (NH3), which is the base of fertilizer, directly from using nitrogen (N2) in the air as an alternative method. They also appear in high school chemistry textbooks.
Furthermore, Setsuro Tamaru played an important role as a member of this Haber group while studying in Germany. After returning to Japan, Tamaru was devoted to the development of Tokyo Institute of Technology and served as the first library director. This achievement was accomplished in 1913, and since it was confirmed in 1914 to 1915 as a paper, it was more than 100 years ago.
After that, at the Tokyo Institute of Technology, groups of Atsumu Ozaki and Kenichi Aika from the latter half of the 1960s to the 1970s, and a group of Professor Emeritus Hideo Hosono have developed a new catalyst for ammonia synthesis and achieved high performance, although it did not inherit Tamaru's flow.
Setsuro Tamaru (1879-1944)
Setsuro on the left in the center and Haber on the right (1913)
(Source) "People who supported humanity through ammonia synthesis" (Tokyo Tech Museum and Archives)