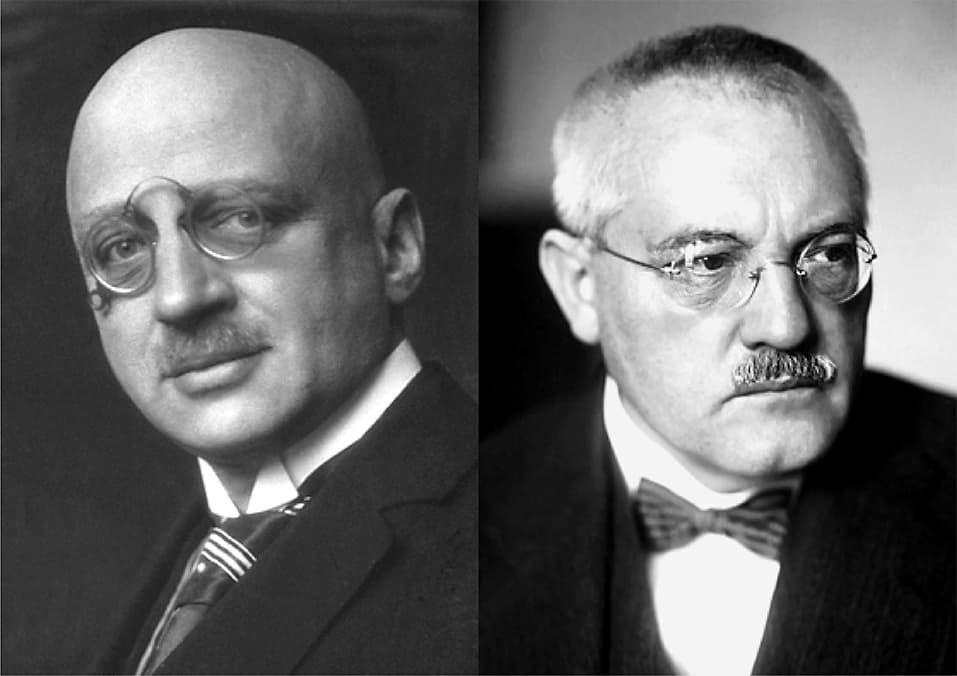What’s Ammonia?
The world's population continues to grow and now exceeds 7 billion. It is ammonia (NH3), a substance with a strong smell, which supports this population increase because nitrogen (N) is essential for the growth of plants such as agricultural products, and ammonia is used as a supply source. Hence, it is no exaggeration to say that without a stable supply of ammonia and a stable supply of agricultural products, the current population cannot exist.
About Ammonia
An inorganic compound whose molecular formula is NH3. It is a colorless gas at room temperature and pressure with a unique strong irritating odor. Since it dissolves well in water, it is often used as an aqueous solution (ammonia solution), which is important as a basic nitrogen source in the chemical industry.
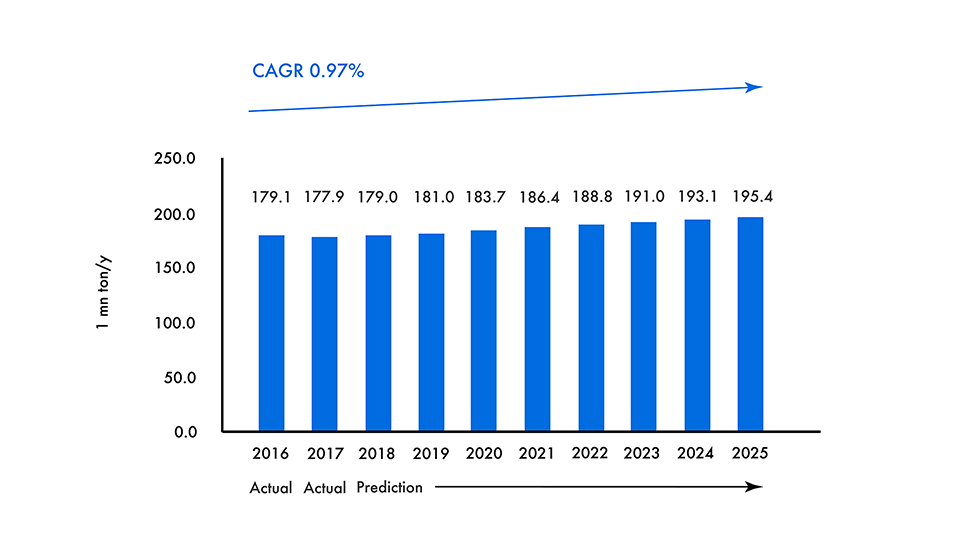
The annual production volume of it in the world is about 180 million tons and it is one of the largest production of chemical products.
It is said that the name comes from the fact that ammonium salts were found near the Temple of Amon in ancient Egypt, and Joseph Priestley (1774) was the first person to synthesize ammonia.
Characteristics of ammonia
Molecular weight: 17.0306
Boiling point: -33.34℃
Freezing point: -77.73℃
Ammonia is classified as flammable gas and toxic to human body. It has a peculiar pungent odor.
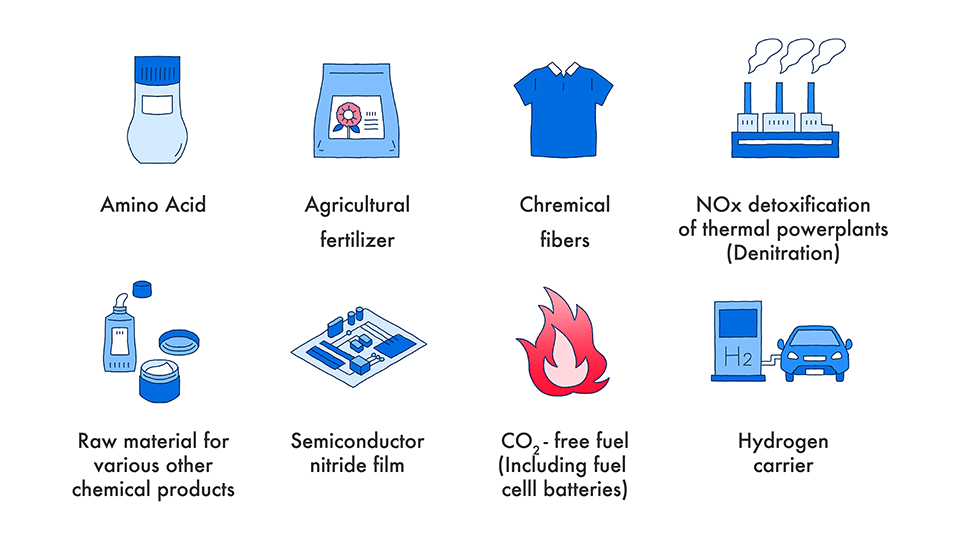
Ammonia has a wide variety of uses.
Fertilizer: It is said that 80% or more of the uses of ammonia are fertilizer uses. Starting from urea, various nitrogen-based fertilizers such as ammonium sulfate, ammonium phosphate, ammonium chloride, ammonium nitrate and potassium nitrate are produced using ammonia as a raw material. In North America, there are many fertilization methods in which liquid ammonia is directly sprinkled onto the soil.
Chemical raw material: It is a raw material for various chemical products containing nitrogen atoms, and is made into resins, food additives, dyes, paints, adhesives, synthetic fibers, synthetic rubbers, fragrances, detergents, etc.
Denitration: It is installed in boilers of thermal power plants to suppress the generation of nitrogen oxides(NOx) that are harmful to the environment.
Fuel for thermal power generation: Ammonia burns depending on conditions, and carbon dioxide does not generate when ammonia is burned. For this reason, technology development using ammonia as fuel for thermal power generation is being carried out.
Energy (hydrogen) carrier: Since liquefying ammonia requires less energy than liquefying hydrogen, it is being studied as one of energy and hydrogen storage or transportation means. In addition, some companies are working on the development of fuel cells that directly extract energy from ammonia.
Various Applications of Ammonia
Movement in the Global Production of Ammonia
Why is ammonia important?
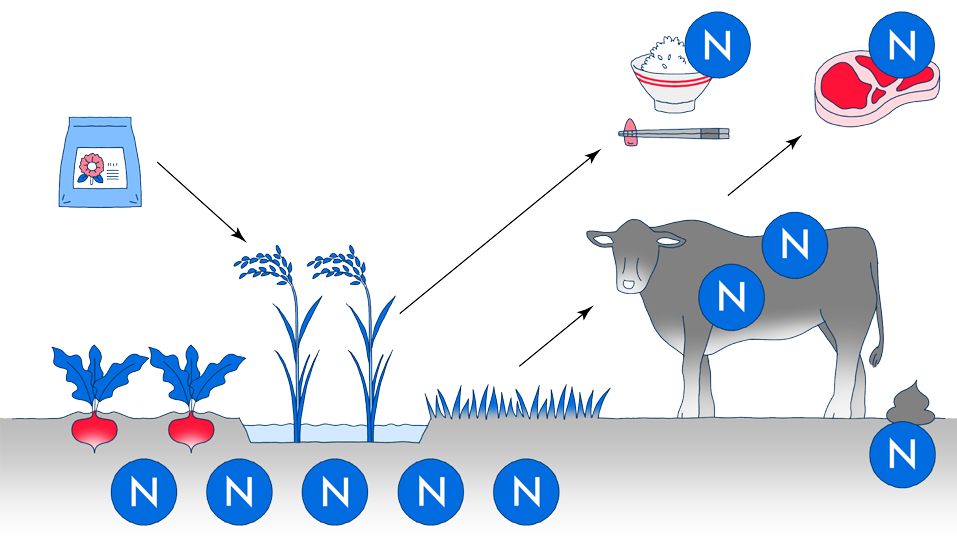
Nitrogen is essential for human beings to survive and it is a constituent of proteins, amino acids, and even DNA; therefore, it is one of the important elements for humans to live.
Approximately 80% of air is made up of nitrogen, but humans cannot directly take in this nitrogen in the air. On the other hand, plants can absorb nitrogen sources (ammonia and nitrogen compounds) contained in soil from their roots. Therefore, by eating crops, humans take in nitrogen into the body and convert it into the substances that are necessary for life.
A large amount of nitrogen is necessary to grow crops sufficiently, so nitrogen fertilizer is indispensable when growing them. For this reason, in the past, the method of mixing nitrogen sources into the soil was used, such as mixing manure with soil, mixing fossils of bird droppings (called guano) with it, and planting legumes to the soil.
However, since the Industrial Revolution, it was not enough to grow those crops to cover the rapidly increasing population, and in the 19th century, research began to artificially synthesize ammonia, which is the source of chemical fertilizers (nitrogen fertilizers).
Today, ammonia is produced around 176 million tons (2016) in the world, and about 80% of it is used as fertilizer, so it can be said to be the backbone of food production in the world.
Model diagram of nitrogen cycle
Haber-Bosch process
Fritz Haber (1868-1934)
Carl Bosch (1874-1940)
Nitrogen is a very stable gas that accounts for about 80% of air.
In other words, “very stable” means it is difficult for plants to decompose and use.
Studies were conducted to artificially synthesize ammonia as a nitrogen fertilizer that is easily taken up by plants from a large amount of stable nitrogen molecules.
With numerous researchers working on it, Fritz Haber first succeeded in synthesizing ammonia from nitrogen gas and hydrogen gas in 1909.
Karl Bosch developed this reaction for industrial use. This enabled large-scale synthesis of ammonia and is still widely used as the Haber-Bosch process.
A key element in this development was the catalyst. Mixing nitrogen gas and hydrogen gas at a ratio of 1:3 does not turn them into ammonia.
In order to synthesize ammonia, the nitrogen molecule bond must be broken, and it must react with hydrogen. This bond is so strong that it is hard to break.
Generally, there is a method of increasing the temperature and pressure, but in the case of nitrogen molecules, it is difficult to break the bond even if it is heated to 800℃. The catalyst facilitates the reaction. The catalyst adsorbs nitrogen molecules and donates electrons to accelerate bond cleavage. The catalyst initially discovered by Haber was based on the rare element osmium (Os). Since expensive osmium is difficult to industrialize, Karl Bosch's colleague Alvin Mittash developed a catalyst that added potassium (K) and aluminum (Al) to the iron (Fe) base and then, Haber Bosch process has been completed. This catalyst has still been in use for more than 100 years since the establishment of the Haber-Bosch process.